Candy-striped spiders Enoplognatha ovata (Clerck, 1757) and Enoplognatha latimana Hippa & Oksala, 1982
Etymology
Enoplognatha - Greek ένοπλος‚ ('enoplos'), armed; Greek γνάθος‚ ('gnathos'), jaw, mandible; ovata - Latin 'ovatus', egg-shaped; latimana - an arbitrary combination of letters to resemble a Latin adjective (H. Hippa, pers. comm.)


Description
Enoplognatha ovata and Enoplognatha latimana are almost identical in size and appearance and can only be separated reliably by the form of the male palp and of the female epigyne in mature individuals[1]. Indeed, just one rather variable species was initially recognized until two Finish arachnologists, Heikki Hippa and Ilkka Oksala, identified E. latimana and E. penelope as separate species within E. ovata[2]. Body lengths range from about 3-5 mm for males and 4-6 mm for females. The cephalothorax is a very light brown with a black rim and a black bar running down the centre (Figure 1); the sternum is similarly marked. The opisthosoma (abdomen) is rather globular in shape with a background colour of yellow/cream. It can, however, have red-pigmented markings superimposed on this background (Figures 2 and 3; see below). There can also be a variable number of black spots forming a line on each dorsolateral flank. These spots are more common in E. ovata than E. latimana but, contrary to some

descriptions, do occur in both species. The ventral surface has a broad black band running from the epigastric fold to the spinnerets and four black spots around the spinnerets (which are constant, irrespective of the presence or absence of dorsal spotting). The legs are unbanded and the colour of the cephalothorax, both of which are darker in mature males.
Distribution and habitat
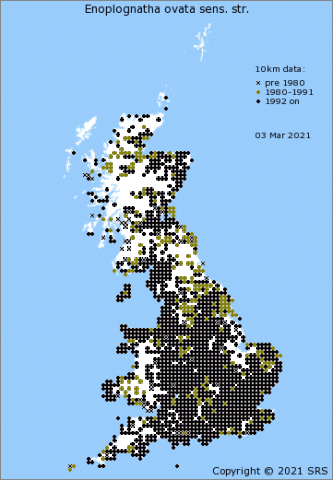
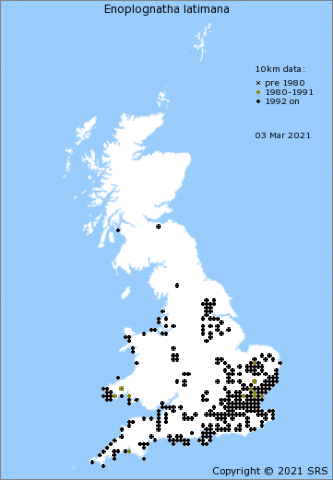
Enoplognatha ovata is one of our commonest, prettiest and most recognizable species of comb-footed spiders (family Theridiidae), found throughout the British Isles (Figure 4[3]) and an occupant of most domestic gardens. In Europe its distribution extends south to the Mediterranean coast, eastwards to the longitude of the Caspian Sea and to the north reaches the southern coasts of Norway, Sweden and Finland [4][5]. E. latimana is found in England, Wales and Ireland, but not in Scotland (Figure 5[6]). When it first started to be mapped in England and Wales the species seemed to be confined to a coastal strip (Lancashire around to East Anglia) but has increasingly been reported inland. Whether this is just a sampling artefact or a result of the species expanding its range isn't yet clear. On the Continent, E. latimana occupies a more southerly distribution than E. ovata, even extending into North Africa [4][7], and is also found further east in Asia[4].
Both species are usually associated with low-growing vegetation and shrubs; occasionally the lower branches of trees. In continental Europe the two species are frequently found occupying the same habitat as mixed-species populations[8]. In the British Isles, however, E. latimana seems to be confined to more open, hotter and drier environments such as sand dunes, coastal cliffs and heathlands (e.g. in the Breckland of East Anglia)[9]. (Other, similar species, such as E. penelope, also occur on the continent.)

General life history
Enoplognatha ovata and E. latimana are strict annuals. Second instar young overwinter at grass-roots level. During the period April to June the young of E. ovata grow and move higher up the vegetation, maturing in late June (males) and early July (females). After mating males soon die and gravid females build retreats by rolling a leaf of plants such as bramble and nettle (Figure 6).
Within this leaf they deposit and guard their single, blue egg sac (Figure 3). Towards the end of August and through September emaciated females wander from their retreats into the general vegetation where they die. This coincides with the emergence of the second-instar young from the egg sac (Figure 7).
The young remain in their natal leaf for a variable period before gradually migrating down to the herb layer to overwinter. The phenology of E. latimana is identical except that all stages are delayed by a few weeks. In one study, E. latimana was, on average, about 1.5 moults behind E. ovata within a single, mixed-species population[8]. Overall reproduction investment in the two species is the same but, within an egg sac, E. ovata lays fewer (means: 110 vs. 152) but larger (mean

diameters: 0.69 mm vs. 0.63 mm) eggs than E. latimana (Oxford, unpub.).
Genetic variation and its implications
Enoplognatha ovata has been the subject of a number of studies over the last 80 years, most of which have concerned the striking colour and pattern variation found in virtually all populations (see (ref.[4]) for a list of historical publications). The variation comprises three major forms (morphs): lineata with a plain yellow/cream opisthosoma, redimita which has, in addition, a pair of dorsolateral carmine stripes and ovata in which the entire dorsal surface is carmine. These morphs are inherited and comprise a genetic polymorphism. The vast majority of populations contain both the lineata and redimita morphs; the ovata morph is more sporadic. Black spotting and when, during development, the red pigments appear in morphs redimita and ovata are also under separate genetic control. All of this genetic variation is also found in E. latimana, implying that it was present in the common ancestor of the two species. The redimita colour morph is often at a lower frequency in E. latimana, and the ovata morph especially so.
Most genetic work has focused on the colour variation in E. ovata in an attempt to elucidate what controls the frequencies of the three morphs within populations. The evidence that the polymorphism is maintained by natural selection is overwhelming, although there can be great variation in morph frequencies between populations only tens of metres apart, some of which is a result of pure chance[10]. The adaptive significance of the polymorphism is unknown but may relate to the way predators search for prey. There are two principal mechanisms that could operate. First, predators might develop a search image for prey with a particular appearance and, at the same time, ignore other prey that look different (a process called apostatic selection). Thus, if lineata is common in a population predators (such as birds) would come across this colour morph frequently and learn to associate it with food. They would actively seek out lineata individuals but at the same time tend to overlook other morphs, such as redimita. So lineata is selected against and falls in frequency and redimita is selected for and becomes more numerous. At some stage, when redimita is very common, predators will switch their search image and actively seek out this morph as food, consequently under-predating lineata. In this way both colour morphs will be maintained in the population but at constantly varying frequencies.
A second mechanism that can select for variability in populations involves dietary wariness. Like humans faced with a bowl of bright purple mashed potato, predators may reject a novel prey item (for example, a colour morph they haven't seen before) for the first few encounters. However, over time, they will get used to this new morph and eventually fully incorporate it into their diets. Computer simulation models[11] show that if novel colour morphs are rejected at the first encounter but from the second encounter onwards are fully incorporated into the diet, very large number of morphs can be maintained.
Remarkably, distantly related species within the family Theridiidae have independently evolved polymorphisms that include colour morphs virtually identical to those exhibited by E. ovata and E. latimana[12]. This is probably a consequence of selection for individuals looking distinct from one another (as explained above) plus developmental constraints on the range of colour patterns available[13].
Geoff Oxford - July 2013
(All photos by Geoff Oxford)
References
- Roberts, M. J. (1995) Collins Field Guide to Spiders of Britain and Northern Europe. Harper Collins, London, UK.
- ↑ Hippa, H. & Oksala, I. 1982. Definition and revision of the Enoplognatha ovata (Clerck) group (Araneae: Theridiidae). Insect Systematics & Evolution, 13: 213-222
- ↑ http://srs.britishspiders.org.uk/portal.php/p/Summary/s/Enoplognatha+ovata+sens.+str
- ↑ to:4.0 4.1 4.2 4.3 Oxford, G. S. & Reillo, P. R. 1994. The world distributions of species within the Enoplognatha ovata group (Araneae: Theridiidae): implications for their evolution and for previous research. Bull. Br. Arachnol. Soc., 9: 226-232. Download pdf of paper.
- ↑ http://www.araneae.unibe.ch/data/951/Enoplognatha_ovata
- ↑ http://srs.britishspiders.org.uk/portal.php/p/Summary/s/Enoplognatha+latimana
- ↑ http://www.araneae.unibe.ch/data/681/Enoplognatha_latimana
- ↑ to:8.0 8.1 Oxford, G. S. & Reillo, P. R. 1993. Trans-continental visible morph-frequency variation at homologous loci in two species of spider, Enoplognatha ovata s.s. and E. latimana. Biol. J. Linn. Soc., 50: 235-253. Download pdf of paper.
- ↑ Oxford, G. S. 1992. Enoplognatha ovata and E. latimana: a comparison of their phenologies and genetics in Norfolk populations. Bull. Br. Arachnol. Soc., 9: 13-18. Download pdf of paper.
- ↑ Oxford, G. S. 2005. Genetic drift within a protected polymorphism: enigmatic variation in color-morph frequencies in the candy-striped spider, Enoplognatha ovata. Evolution, 59: 2170-2184. Download pdf of paper.
- ↑ Franks, D. W. & Oxford, G. S. 2009. The evolution of exuberant visible polymorphisms. Evolution, 63: 2697-2706. Download pdf of paper.
- ↑ http://evolution.berkeley.edu/evolibrary/article/happyface_02
- ↑ Oxford, G. S. (2009) An exuberant, undescribed colour polymorphism in Theridion californicum (Araneae, Theridiidae): implications for a theridiid pattern ground plan and the convergent evolution of visible morphs. Biol. J. Linn. Soc., 96: 23-34.
External links
- Jorgen Lissner's Theridiid pages: http://jorgenlissner.dk/Theridiidae.aspx
- Ed Nieuwenhuys' Araneid pages: http://ednieuw.home.xs4all.nl/Spiders/Theridiidae/Theridiidae.htm
- Czech spiders - E. ovata: http://www.pavouci-cz.eu/Pavouci.php?str=Enoplognatha_ovata
- Czech spiders - E. latimana: http://www.pavouci-cz.eu/Pavouci.php?str=Enoplognatha_latimana
- Spiders of Europe - E. ovata: http://www.araneae.unibe.ch/data/951/Enoplognatha_ovata
- Spiders of Europe - E. latimana: http://www.araneae.unibe.ch/data/681/Enoplognatha_latimana
